Novel cancer vaccination platform technology
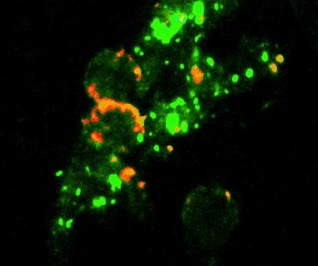
Fig. 1: The CyTuVax vaccine: cytokine aggregates (green)
tumor cells (red) and tumor cell fragments (red).
CyTuVax has developed a powerful vaccination technology consisting of depot-attached cytokines as adjuvant and irradiated autologous tumor cells as vaccine target.
The distinctive feature of our vaccine is the highly improved antigen presentation by dendritic cells (DCs), which results in a strong activation of immune ‘killer’ cells. No other tumor vaccination technology is able to carry cytokine-micro-aggregates directly into the lymph node to improve and boost the activation of the patient’s immune cells. It is this direct stimulation of immune cells in the lymph node of the patient that induces the powerful immune response against the tumor.
The CyTuVax tumor vaccine consists depot-attached aggregated cytokine molecules as adjuvant and irradiated autologous tumor cells and/or specific tumor peptides as vaccine target.
A microscopic detail of the injection-ready CyTuVax vaccine is shown in Fig. 1. The adjuvant consists of several cytokines attached to a depot material in such a way that macro-aggregates are formed. After injection, these cytokine aggregates slowly break down into micro-aggregates and release “bursts” of cytokine aggregates. This release process is the decisive step for the efficiency of the CyTuVax’ vaccine.
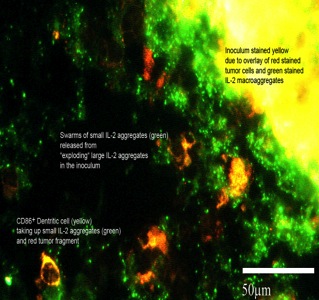
Fig. 2: Cytokine release pattern of the CyTuVax vaccine
Two factors are crucial in the built-up of a powerful and well-targeted anti-tumor immune response:
(1) the specifically engineered release process that mimics the natural release pattern of cytokines by immune cells. When communicating with other immune cells, cytokine molecules are released from vesicles into the cell’s environment. This spray-like release of cytokines attracts an influx of cells of the innate immune system, among others dendritic cells, to the injection site.
Fig.2 shows how these bursts of small cytokine aggregates (small green dots) attract DCs, the main players of immune response induction. With their receptors, the DCs are able to bind these cytokines. The DCs migrate to the nearest lymph node draining the inoculation site to deposit these cytokines.
(2) the release process of cytokines takes place over a long period of time. This means that the vaccines are active for a long period and capable of constantly attracting DCs to the inoculation site.
Inside the lymph nodes, DCs present peptides of the cancer antigen fragments from the autologous tumor cells and deposit cytokine aggregates in the lymph node’s extra cellular matrix. This process forcefully stimulates the T cells and NK cells into action. A powerful immune response is followed as the cytokine-aggregates decompose inside the lymph nodes (see Fig. 3). The specifically activated T cells and NK cells then leave the lymph nodes to search and destroy tumor cells wherever these can be found in the patient’s body.
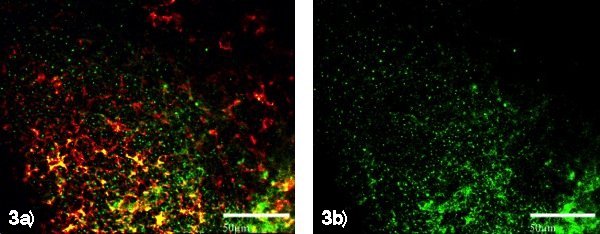
Fig. 3a) CD86+ dendritic cells (red/yellow stain) Fig. 3b) This shows the IL-2 micro-aggregates (green) carried and deposited into the lymph node by dendritic cells.
Contrary to other immunotherapy concepts using in vitro engineered autologous dendritic cells, the attraction and maturation of DCs is achieved in situ, at the inoculation site, under the influence of the released cytokines, and not in vitro in tissue culture. This approach sets the CyTuVax’ technology apart from its competitors and overcomes a major weakness of in vitro stimulated cells: their inability to migrate to the lymph nodes. Our vaccines stimulate the ‘natural’ activation and maturation processes whereby matured DC’s have kept their full migratory powers and reach the lymph node to initiate a powerful anti-tumor response by killer cells.
The CyTuVax vaccination technology is a platform technology in several aspects. It can be applied with a wide range of cytokines and other immune-stimulating agents and against a wide range of tumors.
The vaccine has been successfully tested in preclinical studies with murine model tumors (fibro-sarcoma, melanoma, kidney, colon, mammary carcinoma and B-lymphoma).




